The potential safety hazards of lithium ion batteries (LIBs), as well as their capacity fade and long charging time, are major challenges that currently prevent the widespread adoption of electric vehicles. These effects are strongly correlated with lithium plating, a parasitic reaction on graphite anodes that competes with lithium intercalation when LiBs are charged at high rates or under low temperature. Li plating is known for depletion of the lithium inventory, cell shorting, and thermal runaway.
Addressing Li plating problem relies on material engineering and battery operation optimization. The efficacy of these approaches lies in the fundamental understanding of Li plating mechanism. Despite Li ion battery has been invented for 30 years, a systematic understanding on the onset and growth of Li in graphite anode is still lacking.
Research Highlights: Li plating mechanism in Li-ion battery
we elucidate the mechanism of Li plating, by investigating the interplay between Li intercalation and Li plating. The discovery sheds light on directions and guidelines of materials innovation or electrode design for reducing the risk of Li plating and extreme fast charging. Read more.
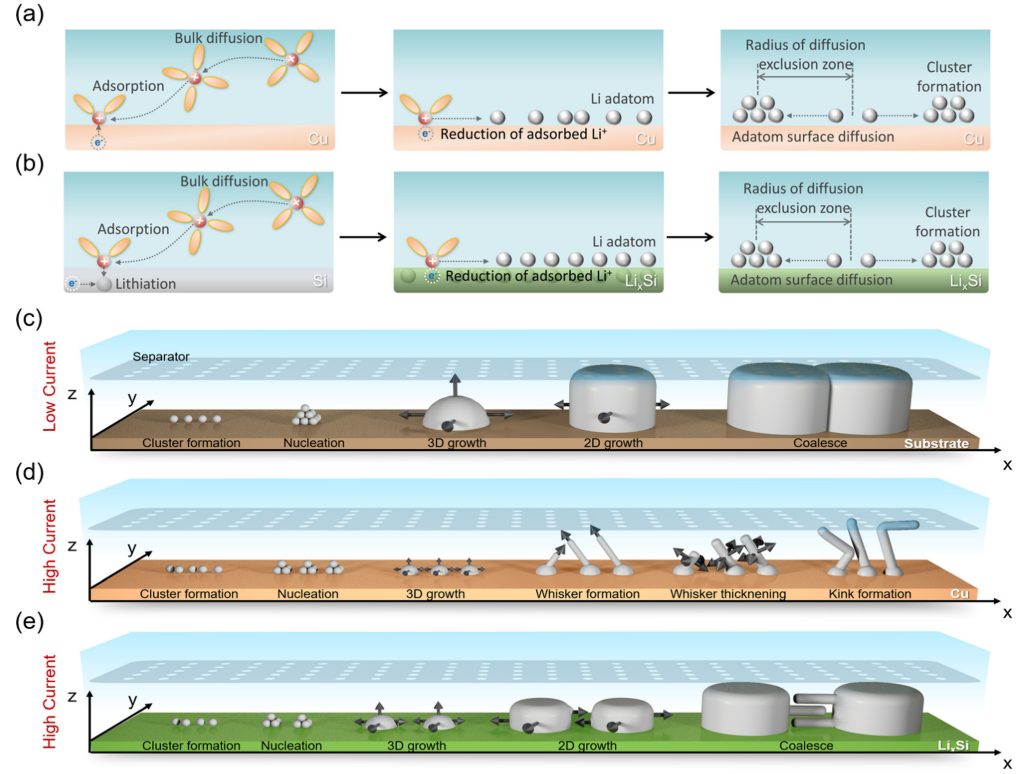
Research Highlights: Li plating mechanism on copper and silicon substrate
Electrochemical Li deposition occurs in fast-charging Li-ion batteries and Li metal batteries. The morphologies of the Li deposits govern the reversibility of the deposition/stripping reaction, and affect the tendency of internal-shorting, therefore determining the performance and safety of Li-ion and Li metal batteries. Many different morphologies, including hemi-sphere, granular, columnar, whisker, and dendrite, have been observed. However, how Li deposits grow into different morphologies largely remains elusive. In this work, we reveal the mechanism of Li growth on Si and Cu substrates in a commercial carbonate electrolyte by combing electron imaging, optical imaging, molecular dynamics simulation and electrochemical tests with theoretical analysis. Li growth dynamics is analyzed by comparing images of Li deposits at different currents, capacities, and temperatures. Read more.
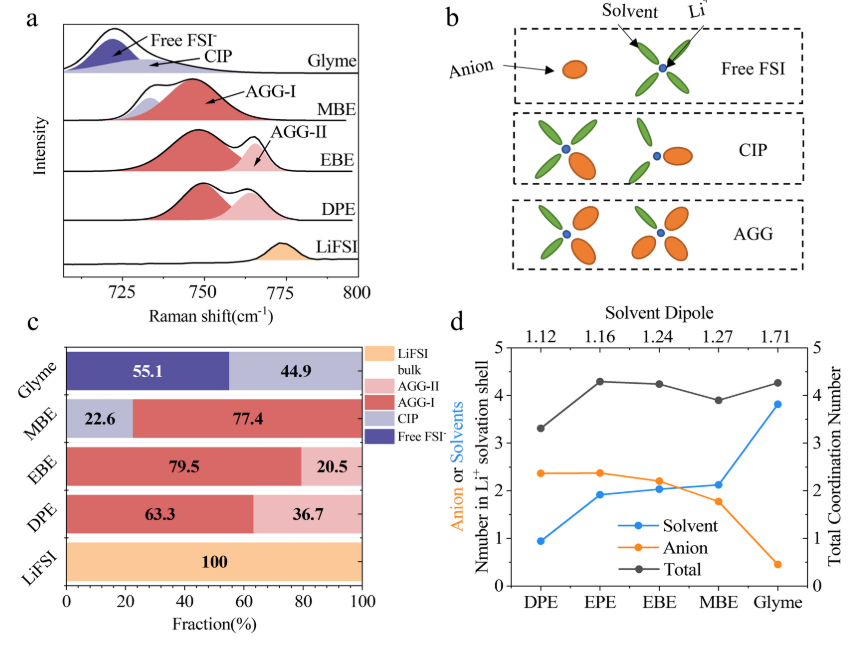
Research Highlights: Electrolytes for fast-charging Li-ion batteries
With the proliferation of electric vehicles, next-generation lithium-ion batteries (LIBs) need to satisfy the requirements of both fast charging and low-temperature operation. As a key component in LIBs, the electrolyte dictates the electrode–electrolyte interfacial properties and the bulk transport properties, thereby crucially governing LIBs' performance. Due to their reasonable polarity, low viscosity and good cathodic stability, ether solvents are widely used in Li metal batteries (Li/S, Li/air, etc.). However, due to their co-intercalation problem, ether solvents are seldom used in LIBs. In this work, we report a series of electrolytes based on single-oxygen linear ether (SOLE), which show great compatibility with the graphite anode at the normal salt concentration (1 m and ∼0.7 M) and better fast-charging and low- temperature performances than commercial electrolytes. Read more.